Answer:
Option A,C,D
Explanation:
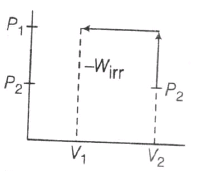
Irreversible Compression
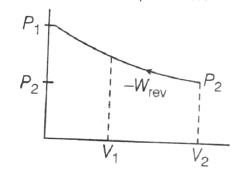
Reversible compression
Maximum work is done on the system when compression occurs irreversible and minimum work is done is reversible compression
(b)
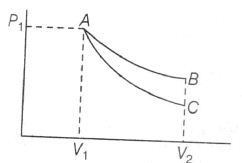
AB is isothermal and AC is the adiabatic path. Work was done in an area under the curve. Hence, less work is obtained in adiabatic process than in isothermal process.
(c) It is incorrect. In adiabatic expansion cooling is observed , hence
$\triangle U=nC_{v}\triangle T<0$
(d) q=0( adibatic) W=0(Free expansion)
Hence Δ U=0 , Δ T=0( isothermal)